13, August 2019
Two new drugs offer hope against Ebola in DR Congo 0
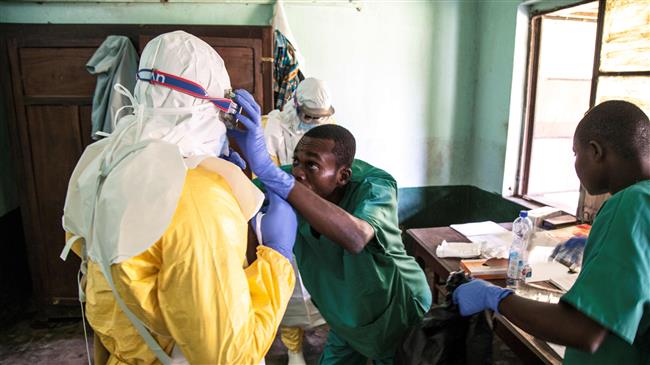
Two experimental Ebola drugs being tested in the Democratic Republic of Congo, where a yearlong outbreak has killed more than 1,800 people, have succeeded in raising the survival rate to around 90%, health authorities said Monday.
Scientists are a step closer to finding the first effective treatments for the deadly Ebola haemorrhagic fever after two potential drugs showed survival rate of as much as 90% in a clinical trial in Congo.
Two experimental drugs – Regeneron’s REGN-EB3 and a monoclonal antibody called mAb114 – were both developed using antibodies harvested from survivors of Ebola infection.
The treatments are now going to be offered to all patients in the Democratic Republic of Congo (DRC), according to U.S. National Institute of Allergy and Infectious Diseases.
They showed “clearly better” results in patients in a trial of four potential treatments being conducted during the world’s second largest Ebola outbreak in history, now entering its second year in DRC.
The drugs improved survival rates from the disease more than two other treatments being tested – ZMapp, made by Mapp Biopharmaceutical, and Remdesivir, made by Gilead Sciences – and those products will be now dropped, said Anthony Fauci, one of the researchers co-leading the trial.
The agency said 49% of the patients on ZMapp and 53% on remdesivir died in the study. In comparison, 29% of the patients on REGN-EB3 and 34% on mAb-114 died.
Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases, told reporters in a telebriefing the results were “very good news” for the fight against Ebola.
“What this means is that we do now have what look like (two) treatments for a disease for which not long ago we really had no approach at all,” he said.
The agency said of the patients who were brought into treatment centres with low levels of virus detected in their blood, 94% who got REGN-EB3 and 89% on mAb114 survived.
In comparison, two-third of the patients who got remdesivir and nearly three-fourth on ZMapp survived.
Ebola has been spreading in eastern Congo since August 2018 in an outbreak that has now become the second largest, killing at least 1,800 people. Efforts to control it have been hampered by militia violence and some local resistance to outside help.
A vast Ebola outbreak in West Africa become the world’s largest ever when it spread through Guinea, Liberia and Sierra Leone from 2013 to 2016 and killed more than 11,300 people.
The Congo treatment trial, which began in November last year, is being carried out by an international research group coordinated by the World Health Organization (WHO).
Mike Ryan, head of the WHO’s emergencies program, said the trial’s positive findings were encouraging but would not be enough on their own to bring the epidemic to an end.
“The news today is fantastic. It gives us a new tool in our toolbox against Ebola, but it will not in itself stop Ebola,” he told reporters.
Jeremy Farrar, director of the Wellcome Trust global health charity, also hailed the success of the trial’s findings, saying they would “undoubtedly save lives”.
“The more we learn about these two treatments, …the closer we can get to turning Ebola from a terrifying disease to one that is preventable and treatable,” he said in a statement.
“We won’t ever get rid of Ebola but we should be able to stop these outbreaks from turning into major national and regional epidemics.”
Some 681 patients at four separate treatment centres in Congo have already been enrolled in the Congo treatment clinical trial, Fauci said. The study aims to enrol a total of 725.
The decision to drop two of the trial drugs was based on data from almost 500 patients, he said, which showed that those who got REGN-EB3 or mAb114 “had a greater chance of survival compared to those participants in the other two arms”.
(REUTERS)


























19, August 2019
Chinese medical team provides free treatment in rural Cameroon 0
In early August, on the eve of the arrival of a medical team with free services, villagers gathered in groups of twos and threes, buzzing with tales of the wondrous Chinese doctors.
“Without them, I would have been dead” said Christophe Ndi Owona, the 76-year-old chief of the southern Cameroonian village of Ngat-Bane.
Before Chinese doctors came to his village, Owona had been suffering from severe headache for years. “Everybody thought I would die.”
The team gave him medicine and advised him to stop drinking alcohol, Owona told his folks. “Thanks to the Chinese, I am now as healthy as a baby.”
Ngat-Bane, like many other Cameroonian villages, has abundant wildlife and dispersed thatched huts, but zero hospitals. Villagers are often troubled by such health problems as rheumatism, typhoid, malaria, but most of them cannot afford medical services in Mbalmayo, the nearest town.
The next day, in the heartland of Ngat-Bane, a good number of patients from nearby villages gathered in a makeshift, but free and comprehensive clinic, lining up to see doctors from various departments, who are here as members of the 19th Chinese medical team to Cameroon.
For the first time in almost a year, 69-year-old Liliane Mfoumou felt relieved from her back injury after an acupuncture treatment. Before this, the mother of six had visited doctors several times in Mbalmayo, a major city in Cameroon’s Central Province.
“Medical checkups and treatments in hospitals are quite expensive, and I can’t afford them again,” Mfoumou said, expressing gratitude to the free clinic.
A Chinese acupuncturist inserted fine needles in Mfoumou’s skin at specific points. Thanks to this traditional Chinese method, which is applicable to many health conditions, Mfoumou said she “now can feel my back and legs.”
Michel Ndi has insomnia and heart problems, making it difficult for the 59-year-old to trek to his farm. He consulted almost all the specialists and was offered free treatments, including medicine, for his multiple conditions.
“Their kind and friendly manner heals you even before you start treatment,” said the father of 10 children, “I am sure that in the next two or three days, I will be able to go (to) the farm without difficulties.”
A total of 100 patients, aged from 20 months to 98 years, received diagnosis and treatments worth 1,500 U. S. dollars.
The free clinic ran for more than five consecutive hours, after which the folks offered the Chinese medical team a local dish called Kpem, a traditional meal made from cassava leaves.
China started to dispatch medical teams to Cameroon in 1975 and hundreds of medical professionals have worked in Cameroon since then.
Source: Xinhuanet